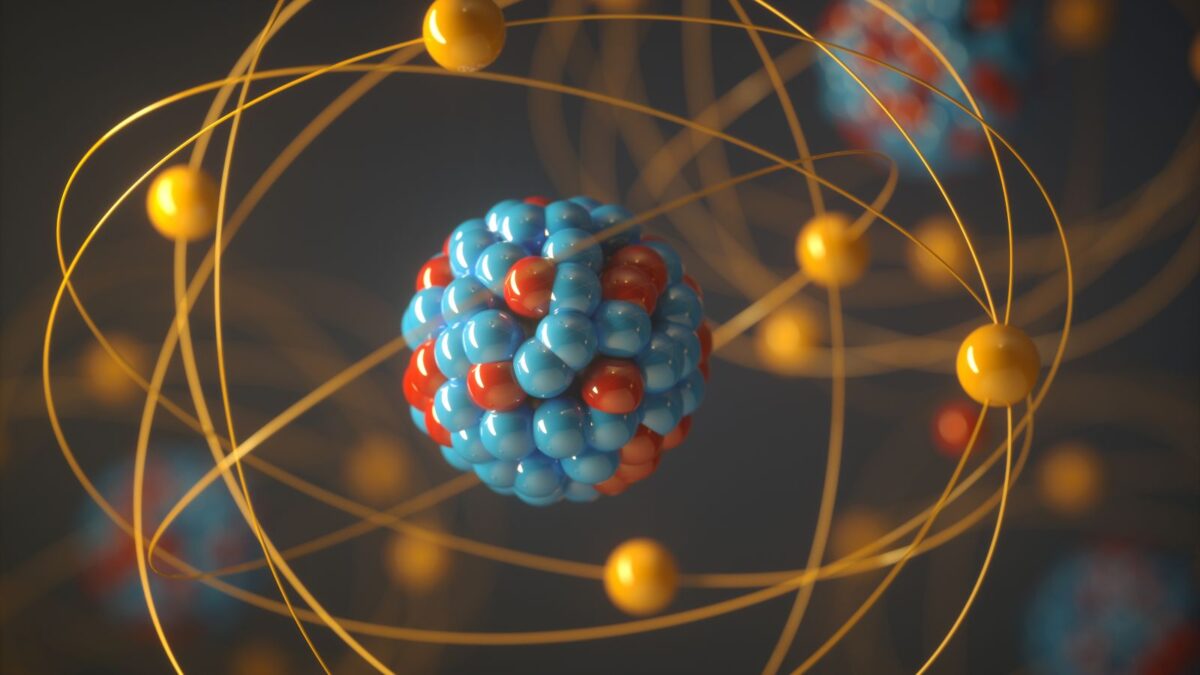
Dive into the microscopic world, where atoms rule and particles play pivotal roles. At the heart of this universe, positively charged particles reside within the atomic nucleus, orchestrating the dance of matter and energy. This article will shed light on these remarkable entities.
Understanding these particles isn’t just for the scientifically minded. It’s a journey into the essence of all things, a peek behind the curtain of reality itself. In today’s interconnected world, Real-Time Communication Solutions provide an example of how advanced understanding and Game Mods technology come together, echoing the intricate ways particles interact. So, buckle up for an exploration into the positively charged particles residing in the atomic nucleus, their characteristics, and their influence on the world we know.
Partikel Bermuatan Positif Yang Terdapat Dalam Inti Atom Adalah
Looking back at scientific history, the exploration of the atom’s structure dates back to the 19th century. It was John Dalton, an English chemist, who first proposed the atomic theory in 1808. His ideas, although rudimentary, laid the groundwork for our current understanding of atomic structure. He suggested that all matter consists of indivisible units called atoms.
Following Dalton, J. J. Thomson, in 1897, discovered particles called electrons through the cathode ray experiments. He suggested that these negatively charged particles are scattered in a balanced, positive charge. Consequently, he proposed the Plum Pudding model, according to which atoms resembled a positively charged sphere comprising miniature negative electrons—like plums embedded in a pudding.
Nevertheless, a major breakthrough came in 1911 with Rutherford’s Gold Foil experiment. It revealed that the Partikel Bermuatan Positif Yang Terdapat Dalam Inti Atom Adalah translated as ‘the positively charged particle located in the atomic nucleus is—,’ a particle that he later termed a “proton.” Rutherford’s study showed that these protons reside at the centre, constituting the nucleus of an atom, something that was not part of Thomson’s previous model.
Partikel Bermuatan Positif dalam Inti Atom: The Proton
Dwelling deeper into the microscopic world, the nucleus houses Partikel Bermuatan Positif Yang Terdapat Dalam Inti Atom Adalah or in English, ‘the positively charged particle located in the atomic nucleus is,’ which is known as the proton. A defining attribute of an element, protons bear significant influence in atomic behavior.

Each atom contains an equal number of protons and electrons, maintaining a neutral charge overall. These protons define the atomic number and hence the chemical identity of an element. For instance, an atom with one proton is always helium, while an atom with two protons corresponds to helium.
Ernest Rutherford, in his Gold Foil experiment, detected these positively charged particles within the atomic core. This experiment marked a significant revelation about atomic structure, highlighting the proton’s position within the nucleus. Accumulated evidence corroborates that protons make up approximately 50% of an atom’s mass, with neutrons accounting for the balance.
The Importance of Protons in Atomic Structure
Advancing from the core principles of atomic structure, the positive particles found in atomic nuclei, or protons, take center stage. An atomic structure owes its unique identity to protons, particles carrying a positive charge. These key components affirm an atom’s elemental identity and help to maintain the balance of charges within the atom.

Existing within the atomic nucleus, together with neutrons, protons leave an undeniable influence on the atom’s identity but also its stability. Looking from the atomic number perspective, it’s mainly the proton count that sets apart one element from another. For example, an atom with 6 protons will always represent Carbon, regardless of how many neutrons or electrons it has.
A shift in the proton count can transmute an atom into a different element, an absolutely crucial point for understanding nuclear chemistry and radioactive decay, where various atomic modifications occur. Furthermore, to maintain electrical neutrality in an atom, the number of protons is typically equal to the number of electrons, thus confirming the criticality of protons in an atom’s charge balance.